vEUA provides a clear, interactive visualisation of complex disease, making it easier for surgeons and radiologists to plan procedures, share findings with colleagues, and communicate with patients.
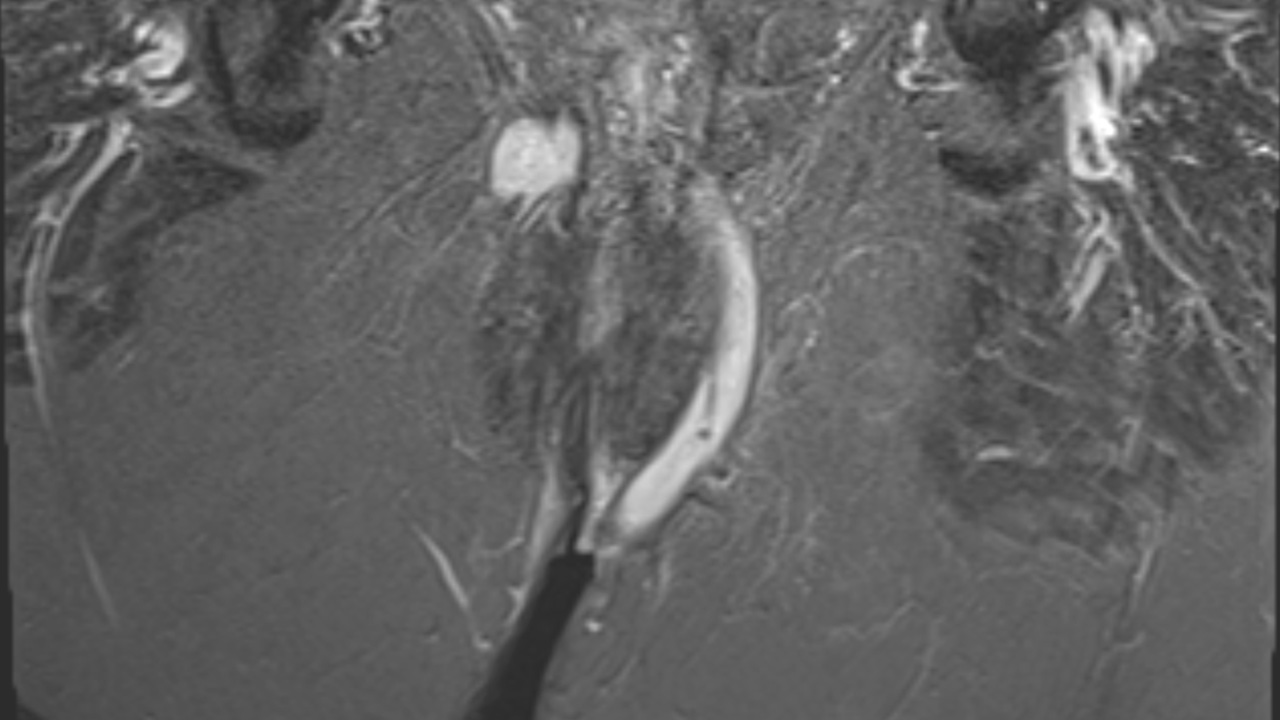
Virtual Examination Under Anaesthetic (vEUA) enables clinicians to build an anatomically representative model of the sphincters, levator plate, and fistula tracts from MRI in under two minutes.
vEUA provides a clear, interactive visualisation of complex disease, making it easier for surgeons and radiologists to plan procedures, share findings with colleagues, and communicate with patients.
Conventional written MRI reports can be difficult to interpret, particularly for complex perianal fistula and pelvic disease. Surgeons and radiologists need a way to communicate anatomy clearly and efficiently with each other and with patients.
vEUA reduces the need for long descriptive passages with a clear, interactive 3D model. This makes it easier to communicate findings, plan procedures, and involve patients in understanding their disease and treatment options.
Outside of surgical planning, the volumetry of the fistula tract and surrounding tissue (including length and branching of the tracts) can be measured with Entrolytics standard tooling.
For complex fistula, anatomy alone is not enough. pTRACK provides:
To visualise fistula tracts and share findings.
To plan procedures and improve communication with radiologists and patients
To guide complex case discussions
To better understand pelvic anatomy
vEUA works directly with pelvic MRI datasets, using standard DICOM imaging.
vEUA models can be accessed and shared securely via Entrolytics, providing interactive 3D visualisation alongside conventional images.
Models can be extended with quantitative disease assessment (volume, tortuosity, signal change, fibrosis) and validated indices (MAGNIFI-CD, Van Assche).
No. vEUA is not yet FDA cleared or CE marked. It is currently available for investigational use in collaboration with research partners.
No. vEUA is not an AI. It relies on the skill of the user to identify anatomy and trace fistula tracts. The software provides tools to rapidly generate anatomically representative models and mark up tracts semi-automatically.
In under 2 minutes, a skilled clinical professional can generate a 3D model of the pelvic anatomy from MRI.
vEUA was developed for surgical and medical management of simple and complex perianal fistula, particularly in Crohn’s disease. It is also increasingly used in colorectal cancer surgery where richer anatomical information supports complex operative planning.
vEUA is not yet FDA cleared or CE marked, it's currently available for investigational use in collaboration with research partners.
Motilent is working with clinical collaborators to gather evidence to support regulatory submissions.
Request a demo - see how models are created in under 2 minutes.
Collaborate on research - join our clinical partners evaluating vEUA in Crohn’s disease and colorectal cancer.
Contact us - find out more about investigational use and future availability.