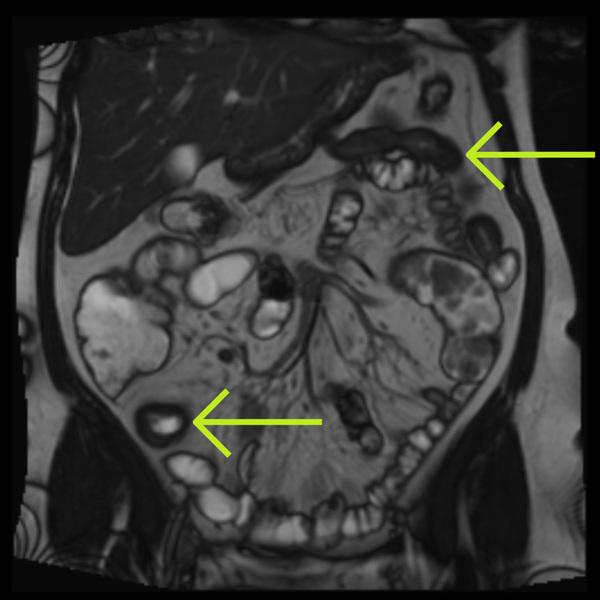
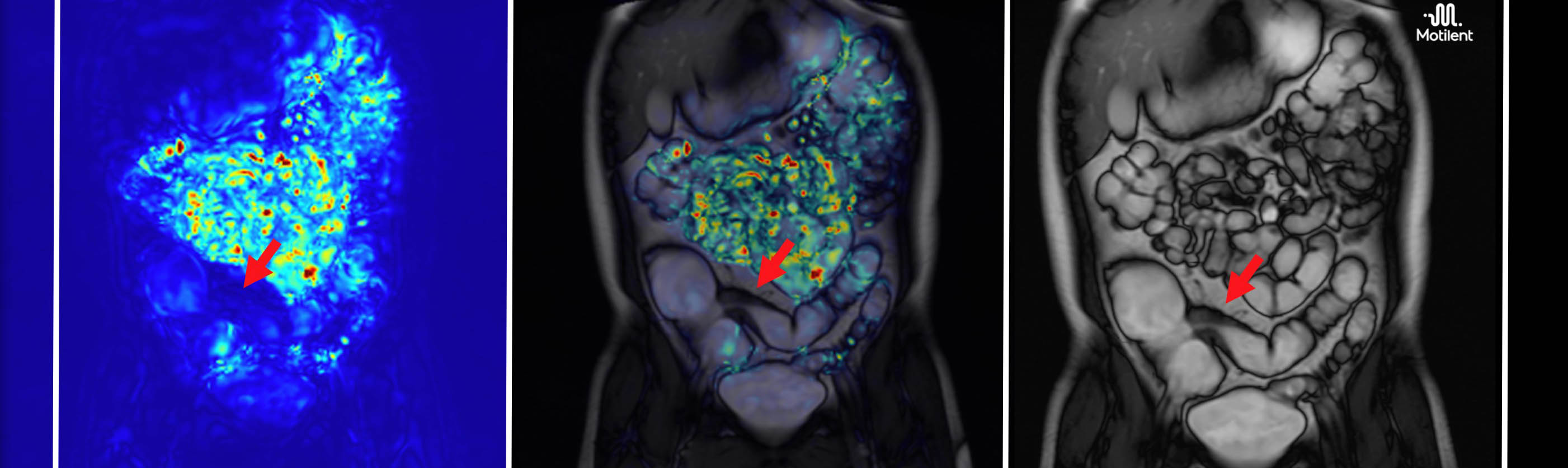
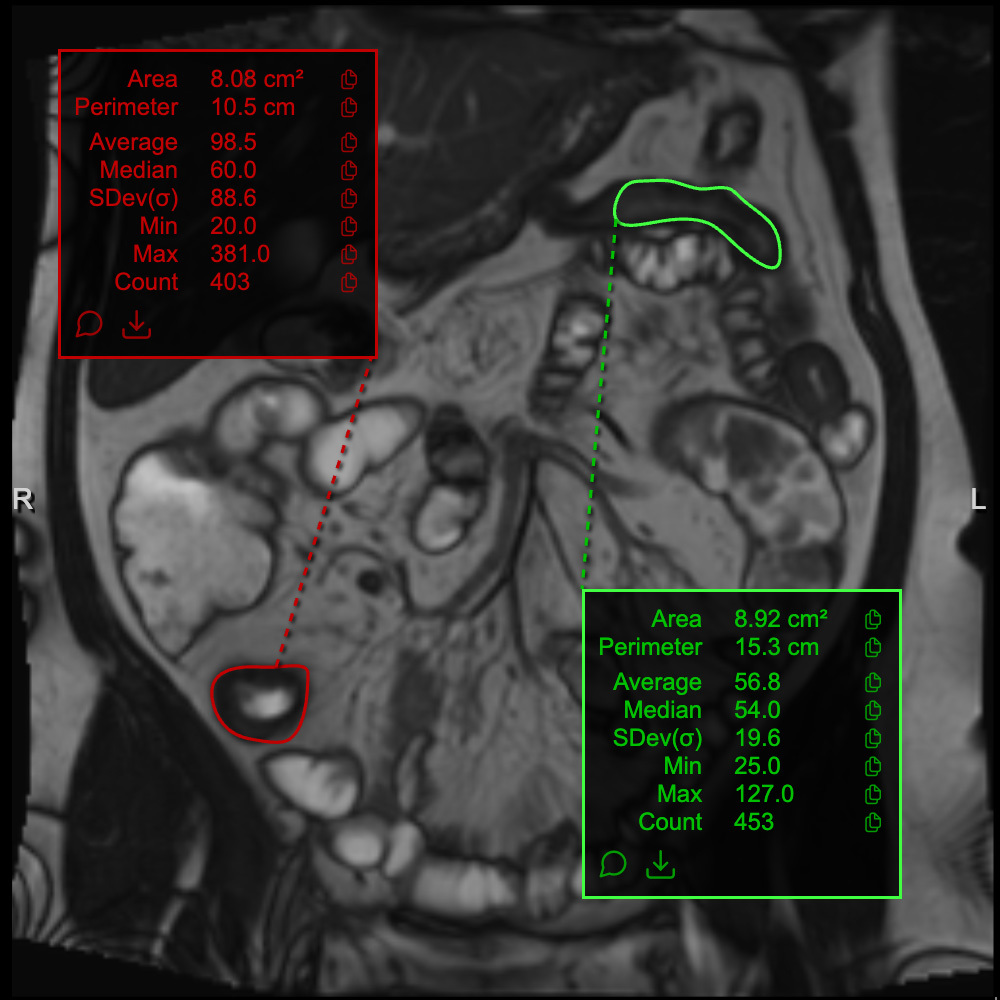
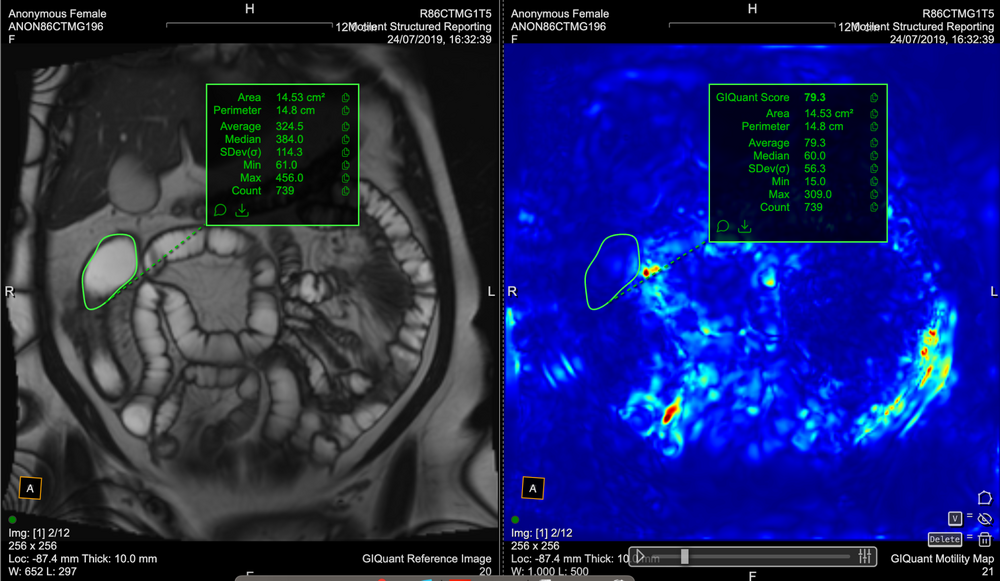
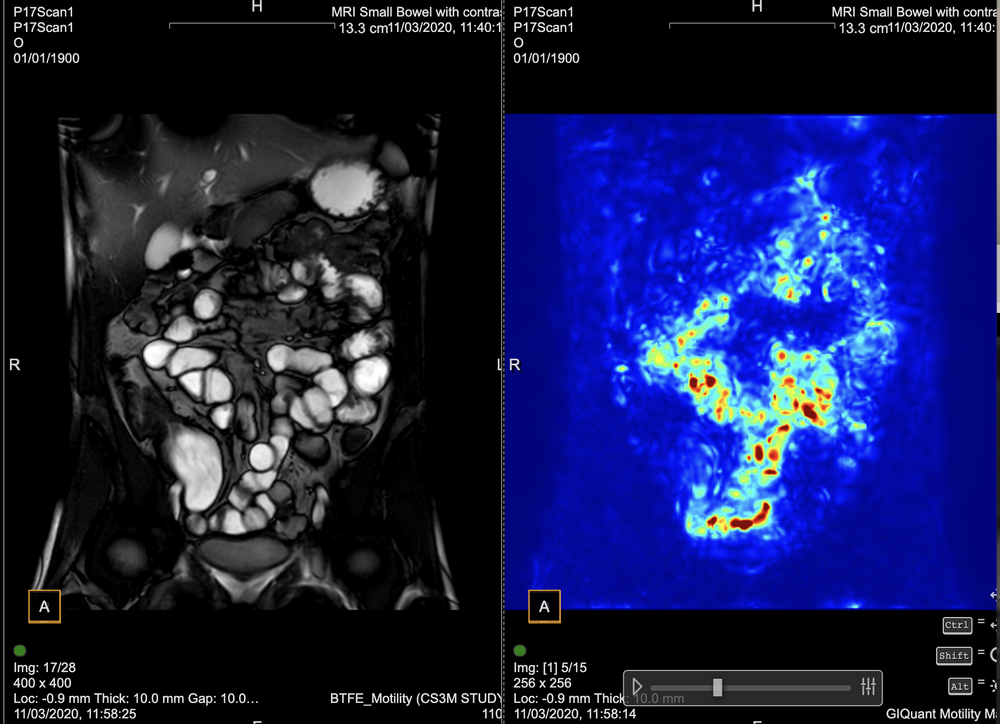
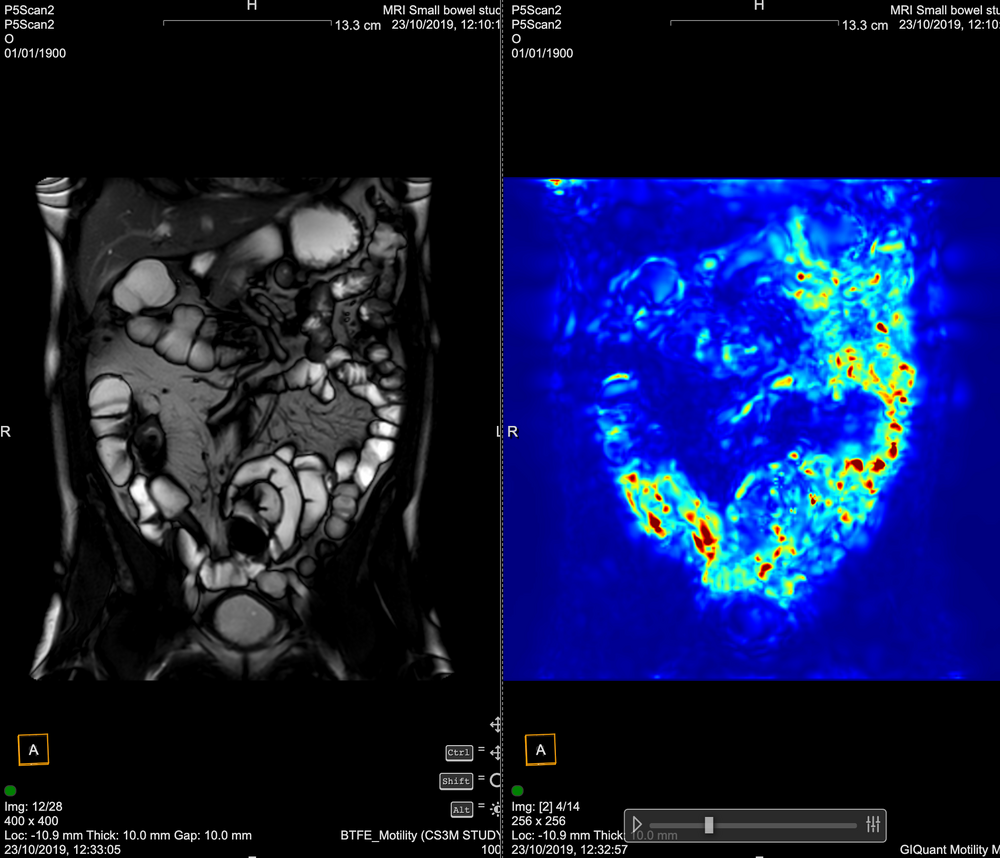
Conventional MRI assessment of Crohn’s disease is largely subjective and relies on visible structural changes such as wall thickening, ulceration or strictures. These changes often appear late in the disease process, meaning clinicians may miss early treatment response or disease progression.
While MRI is a powerful test for showing where the disease is (small bowel vs colon) and what the disease is doing (penetrating or stricturing), it cannot, without the help of measurement tooling, show how the disease is responding, for example to therapy or intervention.
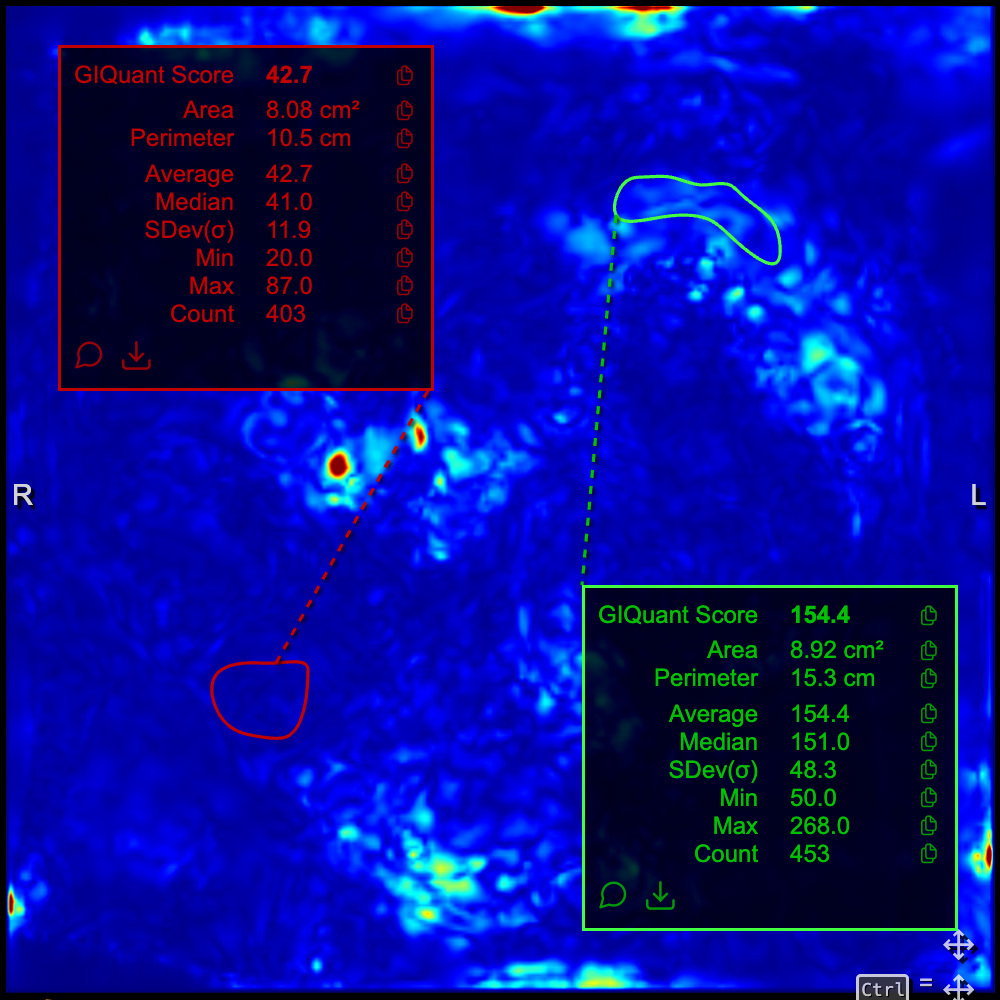
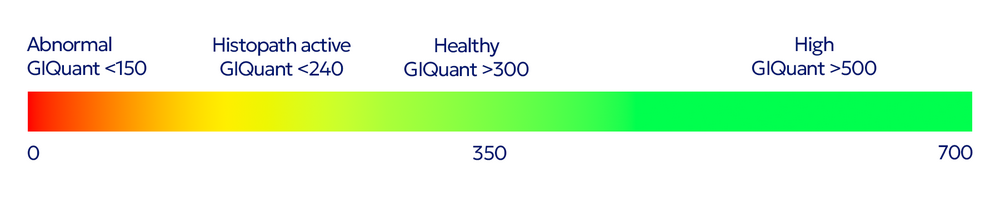
GIQuant provides clinicians with a rapid, objective score for small bowel motility, reducing variability from subjective image interpretation. It helps support standardisation of reads across hospitals and clinical trials.